Ecosyste.ms: Awesome
An open API service indexing awesome lists of open source software.
https://github.com/merck/metalite.ae
An R package for standard adverse events analysis
https://github.com/merck/metalite.ae
cdisc clinical-trials metadata r-package
Last synced: 2 months ago
JSON representation
An R package for standard adverse events analysis
- Host: GitHub
- URL: https://github.com/merck/metalite.ae
- Owner: Merck
- License: gpl-3.0
- Created: 2022-04-19T16:51:48.000Z (almost 3 years ago)
- Default Branch: main
- Last Pushed: 2024-04-15T04:12:02.000Z (10 months ago)
- Last Synced: 2024-04-22T03:14:51.056Z (9 months ago)
- Topics: cdisc, clinical-trials, metadata, r-package
- Language: R
- Homepage: http://merck.github.io/metalite.ae/
- Size: 7.28 MB
- Stars: 14
- Watchers: 7
- Forks: 3
- Open Issues: 2
-
Metadata Files:
- Readme: README.md
- License: LICENSE.md
Awesome Lists containing this project
README
# metalite.ae 
[](https://app.codecov.io/gh/Merck/metalite.ae?branch=main)
[](https://github.com/Merck/metalite.ae/actions/workflows/R-CMD-check.yaml)
[](https://cran.r-project.org/package=metalite.ae)
[](https://cran.r-project.org/package=metalite.ae)
## Installation
The easiest way to get metalite.ae is to install from CRAN:
```r
install.packages("metalite.ae")
```
Alternatively, to use a new feature or get a bug fix,
you can install the development version of metalite.ae from GitHub:
```r
# install.packages("remotes")
remotes::install_github("Merck/metalite.ae")
```
## Overview
metalite.ae is an R package designed for the analysis of adverse events (AE)
in clinical trials.
It operates on ADaM datasets and adheres to the metalite structure.
The R package streamlines the process of generating production-ready tables,
listings, and figures as outlined in the
[AE summary chapter](https://r4csr.org/tlf-ae-summary.html) and the
[specific AE chapter](https://r4csr.org/tlf-ae-specific.html) of the
_R for Clinical Study Reports and Submission_ book.
The package encompasses the following components:
#### AE summary
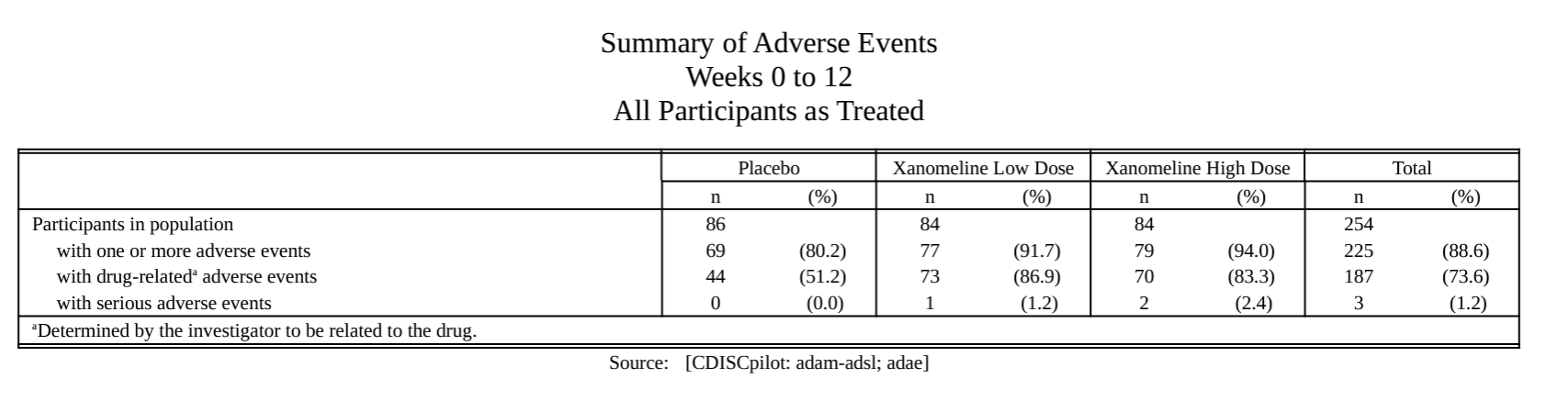
#### Specific AE analysis
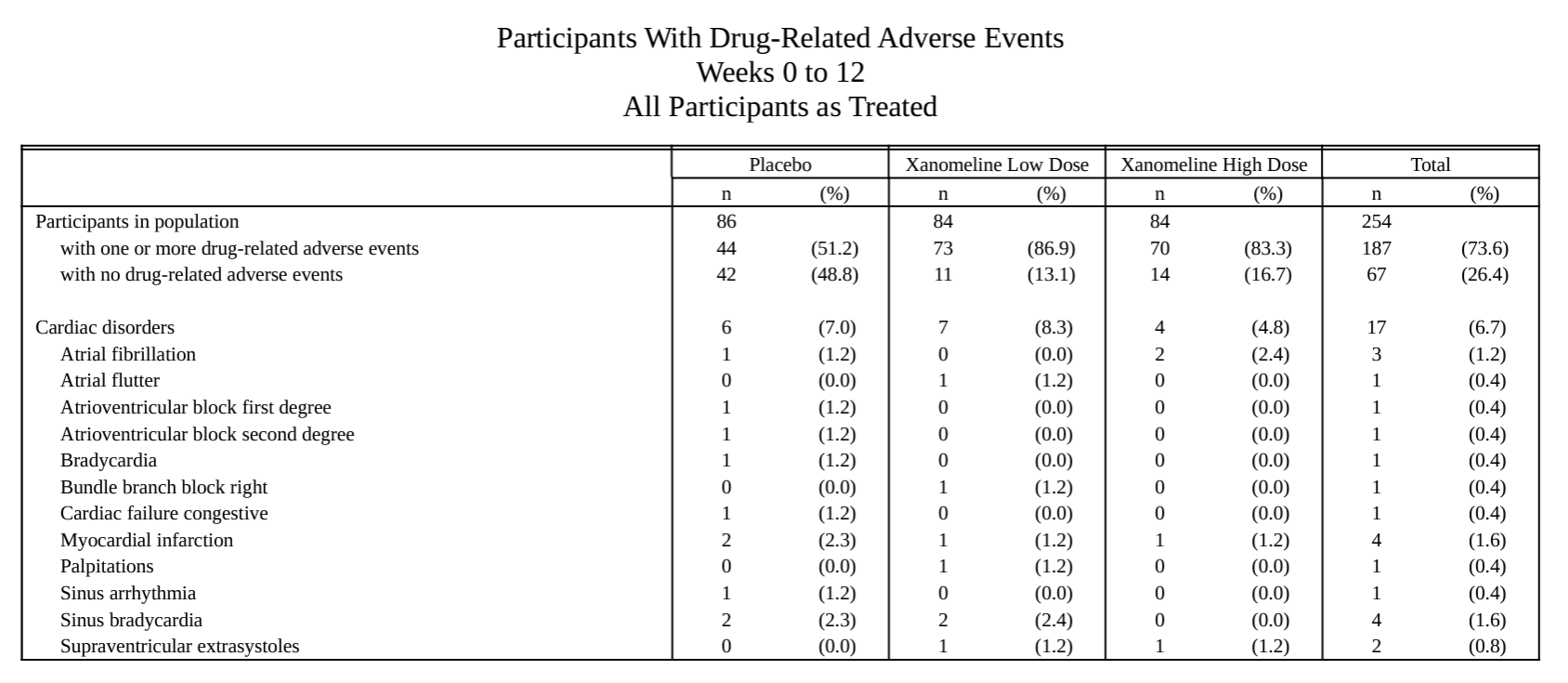
#### AE listing
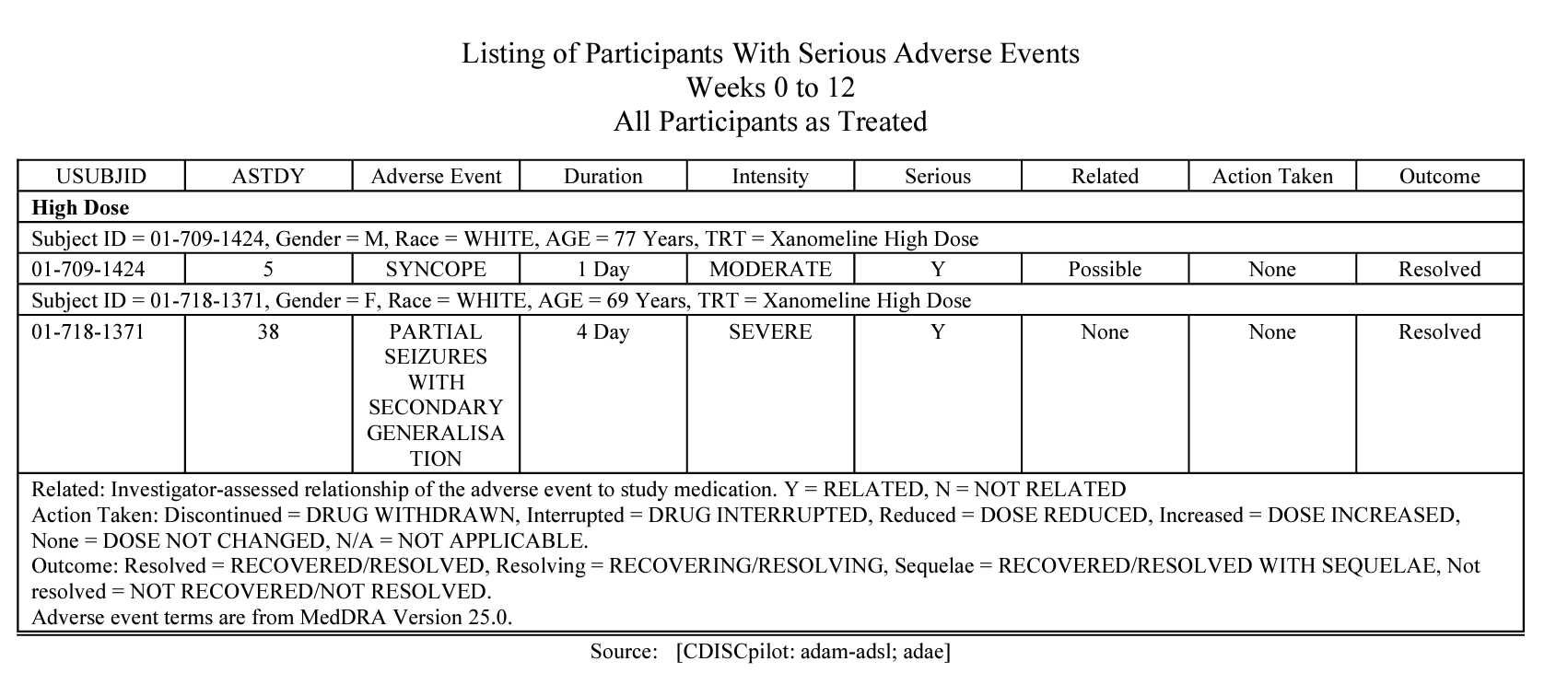
## Highlighted features
- Avoid duplicated input by using metadata structure.
- For example, define analysis population once to use
in all adverse events analysis.
- Consistent input and output in standard functions.
- Streamlines mock table generation.
## Example
```r
meta_ae_example() |> # Example AE data created using metalite
prepare_ae_summary(
population = "apat", # Select population by keywords
observation = "wk12", # Select observation by keywords
parameter = "any;rel;ser" # Select AE terms by keywords
) |>
format_ae_summary() |>
tlf_ae_summary(
source = "Source: [CDISCpilot: adam-adsl; adae]", # Define data source
path_outtable = "ae0summary.rtf" # Define output
)
```
- [Additional tutorials](https://merck.github.io/metalite.ae/articles/metalite-ae.html)