https://github.com/steineggerlab/metabuli
Metabuli: specific and sensitive metagenomic classification via joint analysis of DNA and amino acid.
https://github.com/steineggerlab/metabuli
bioinformatics k-mer metagenomics taxonomic-classification taxonomy
Last synced: 6 months ago
JSON representation
Metabuli: specific and sensitive metagenomic classification via joint analysis of DNA and amino acid.
- Host: GitHub
- URL: https://github.com/steineggerlab/metabuli
- Owner: steineggerlab
- License: gpl-3.0
- Created: 2020-09-09T05:04:49.000Z (about 5 years ago)
- Default Branch: master
- Last Pushed: 2025-03-10T06:06:09.000Z (7 months ago)
- Last Synced: 2025-03-10T07:22:15.440Z (7 months ago)
- Topics: bioinformatics, k-mer, metagenomics, taxonomic-classification, taxonomy
- Language: C++
- Homepage:
- Size: 18.1 MB
- Stars: 130
- Watchers: 6
- Forks: 10
- Open Issues: 16
-
Metadata Files:
- Readme: README.md
- License: LICENSE
Awesome Lists containing this project
README
[](http://bioconda.github.io/recipes/metabuli/README.html)

# Metabuli
***Metabuli*** classifies metagenomic reads by comparing them to reference genomes. You can use Metabuli to profile the taxonomic composition of your samples or to detect specific (pathogenic) species.
***Sensitive and Specific.*** Metabuli uses a novel k-mer structure, called *metamer*, to analyze both amino acid (AA) and DNA sequences. It leverages AA conservation for sensitive homology detection and DNA mutations for specific differentiation between closely related taxa.
***A laptop is enough.*** Metabuli operates within user-specified RAM limits, allowing it to search any database that fits in storage. A PC with 8 GiB of RAM is sufficient for most analyses.
***A few clicks are enough.*** Metabuli App is now available [here](https://github.com/steineggerlab/Metabuli-App). With just a few clicks, you can run Metabuli and browse the results with Sankey and Krona plots on your PC.
***Short reads, long reads, and contigs.*** Metabuli can classify all types of sequences.
---
For more details, please see
[Nature Methods](https://www.nature.com/articles/s41592-024-02273-y),
[PDF](https://www.nature.com/articles/s41592-024-02273-y.epdf?sharing_token=je_2D5Su0-xVOSjuKSAXF9RgN0jAjWel9jnR3ZoTv0M7gE7NDF_xi_3sW8QdRiwfSJNwqaXItSoeCvr7cvcoQxKLt0oROgWc6urmki9tP80cXEuHPN0D7b4y9y3i8Yv7sZw8MxxhAj7W6p9eZE2zaK3eozdOkXvwADVfso9cXIM%3D),
[bioRxiv](https://www.biorxiv.org/content/10.1101/2023.05.31.543018v2), or [ISMB 2023 talk](https://www.youtube.com/watch?v=vz2fuRcVwyk).
Please cite: [Kim J, Steinegger M. Metabuli: sensitive and specific metagenomic classification via joint analysis of amino acid and DNA. Nature Methods (2024).](https://doi.org/10.1038/s41592-024-02273-y)
---
### 🖥️ [Metabuli App](https://github.com/steineggerlab/Metabuli-App) for Windows, MacOS, and Linux are now available!
> Run taxonomic profiling in just a few clicks and explore results with Sankey and Krona plots.
> Download the app for your OS [here](https://github.com/steineggerlab/Metabuli-App/releases)—no separate Metabuli installation needed.
---
### Update in v1.1.0
- Fix errors in v1.0.9
- Custom DB creation became easier
- Improve `updateDB` command
### Update in v1.0.9
- DB creation process improved
- `updateDB` module to add new sequences to an existing database.
- Users can provide CDS information to skip Prodigal's gene prediction.
- `--max-ram` parameter added to `build` module.
- Compatibility with taxdump files generated using [taxonkit](https://bioinf.shenwei.me/taxonkit/).
- Please check release note for details.
### Update in v1.0.8
- Added `extract` module to extract reads classified into a certain taxon.
### Update in v1.0.7
- **Metabuli became faster 🚀**
- Windows: *8.3* times faster
- MacOS: *1.7* times faster
- Linux: *1.3* times faster
- Test details are in release note.
- Fixed a bug in score calculation that could affect classification results.
---
## Table of Contents
- [Installation](#installation)
- [Precompiled binaries](#precompiled-binaries)
- [Compile from source code](#compile-from-source-code)
- [Pre-built databases](#pre-built-databases)
- [Classification](#classification)
- [Extract](#extract)
- [GTDB-based custom database](#gtdb-based-custom-database)
- [Creat a new database](#creat-a-new-database)
- [Add new sequences to an existing database](#add-new-sequences-to-an-existing-database)
- [NCBI or custom taxonomy based database](#ncbi-or-custom-taxonomy-based-database)
- [Creat a new database](#creat-a-new-database-1)
- [Add new sequences to an existing database](#add-new-sequences-to-an-existing-database-1)
- [Example](#example)
## Installation
### Precompiled binaries
```
# install via conda
conda install -c conda-forge -c bioconda metabuli
# Linux AVX2 build (fast, recommended for most Linux system
# check using: cat /proc/cpuinfo | grep avx2)
wget https://mmseqs.com/metabuli/metabuli-linux-avx2.tar.gz; tar xvzf metabuli-linux-avx2.tar.gz; export PATH=$(pwd)/metabuli/bin/:$PATH
# Linux SSE2 build (slower, for old systems)
wget https://mmseqs.com/metabuli/metabuli-linux-sse2.tar.gz; tar xvzf metabuli-linux-sse2.tar.gz; export PATH=$(pwd)/metabuli/bin/:$PATH
# MacOS (Universal, works on Apple Silicon and Intel Macs)
wget https://mmseqs.com/metabuli/metabuli-osx-universal.tar.gz; tar xvzf metabuli-osx-universal.tar.gz; export PATH=$(pwd)/metabuli/bin/:$PATH
```
Metabuli also works on Linux ARM64 and Windows systems. Please check [https://mmseqs.com/metabuli](https://mmseqs.com/metabuli) for static builds for other architectures.
### Compile from source code
```
git clone https://github.com/steineggerlab/Metabuli.git
cd Metabuli
mkdir build && cd build
cmake -DCMAKE_BUILD_TYPE=Release ..
make -j 16
```
The built binary can be found in `./build/src`.
---
## Pre-built databases
You can download [pre-built databases](https://metabuli.steineggerlab.workers.dev/) using `databases` workflow.
NOTE: The `databases` workflow may not work if you don't use the latest version of Metabuli.
In that case, please manually download databases from this [link](https://metabuli.steineggerlab.workers.dev/).
```
Usage:
metabuli databases DB_NAME OUTDIR tmp
# NOTE
- A human genome (T2T-CHM13v2.0) is included in all databases below.
1. RefSeq Virus (8.1 GiB)
- NCBI RefSeq release 223 virus genomes
- Database will be in OUT_DIR/refseq_virus
metabuli databases RefSeq_virus OUT_DIR tmp
2. RefSeq Prokaryote and Virus (115.6 GiB)
- RefSeq prokaryote genomes (Complete Genome/Chromosome, 2024-03-26) + RefSeq Virus above.
- Database will be in OUT_DIR/refseq_prokaryote_virus
metabuli databases RefSeq OUTDIR tmp
3. GTDB (101 GiB)
- GTDB 214.1 (Complete Genome/Chromosome, CheckM completeness > 90 and contamination < 5).
- Database will be in OUT_DIR/gtdb
metabuli databases GTDB OUTDIR tmp
4. RefSeq Releases 224 (619 GiB)
- Viral and prokaryotic genomes of RefSeq release 224.
metabuli databases RefSeq_release OUTDIR tmp
```
Downloaded files are stored in `OUTDIR/DB_NAME` directory, which can be provided for `classify` module as `DBDIR`.
---
## Classification
```
metabuli classify [options]
- INPUT : FASTA/Q file of reads you want to classify. (gzip supported)
- DBDIR : The directory of reference DB.
- OUTDIR : The directory where the result files will be generated.
- Job ID: It will be the prefix of result files.
# Paired-end
metabuli classify read_1.fna read_2.fna dbdir outdir jobid
# Single-end
metabuli classify --seq-mode 1 read.fna dbdir outdir jobid
# Long-read
metabuli classify --seq-mode 3 read.fna dbdir outdir jobid
* Important parameters:
--threads : The number of threads used (all by default)
--max-ram : The maximum RAM usage. (128 GiB by default)
--min-score : The minimum score to be classified
--min-sp-score : The minimum score to be classified at or below species rank.
--taxonomy-path: Directory where the taxonomy dump files are stored. (DBDIR/taxonomy by default)
--accession-level : Set 1 to use accession level classification (0 by default).
It is available when the DB is also built with accession level taxonomy.
```
- Paratemers for precision mode (Metabuli-P)
- Illumina short reads: `--min-score 0.15 --min-sp-score 0.5`
- PacBio HiFi reads: `--min-score 0.07 --min-sp-score 0.3`
- PacBio Sequel II reads: `--min-score 0.005`
- ONT reads: `--min-score 0.008`
This will generate three result files: `JobID_classifications.tsv`, `JobID_report.tsv`, and `JobID_krona.html`.
> Sankey diagram is available in the [GUI app](https://github.com/steineggerlab/Metabuli-App).
#### JobID_classifications.tsv
1. Classified or not
2. Read ID
3. Taxonomy identifier
4. Effective read length
5. DNA level identity score
6. Classification Rank
7. List of "taxID : k-mer match count"
```
1 read_1 2688 294 0.627551 subspecies 2688:65
1 read_2 2688 294 0.816327 subspecies 2688:78
0 read_3 0 294 0 no rank
```
#### JobID_report.tsv
The proportion of reads that are assigned to each taxon.
```
33.73 77571 77571 0 no rank unclassified
66.27 152429 132 1 no rank root
64.05 147319 2021 8034 superkingdom d__Bacteria
22.22 51102 3 22784 phylum p__Firmicutes
22.07 50752 361 22785 class c__Bacilli
17.12 39382 57 123658 order o__Bacillales
15.81 36359 3 126766 family f__Bacillaceae
15.79 36312 26613 126767 genus g__Bacillus
2.47 5677 4115 170517 species s__Bacillus amyloliquefaciens
0.38 883 883 170531 subspecies RS_GCF_001705195.1
0.16 360 360 170523 subspecies RS_GCF_003868675.1
```
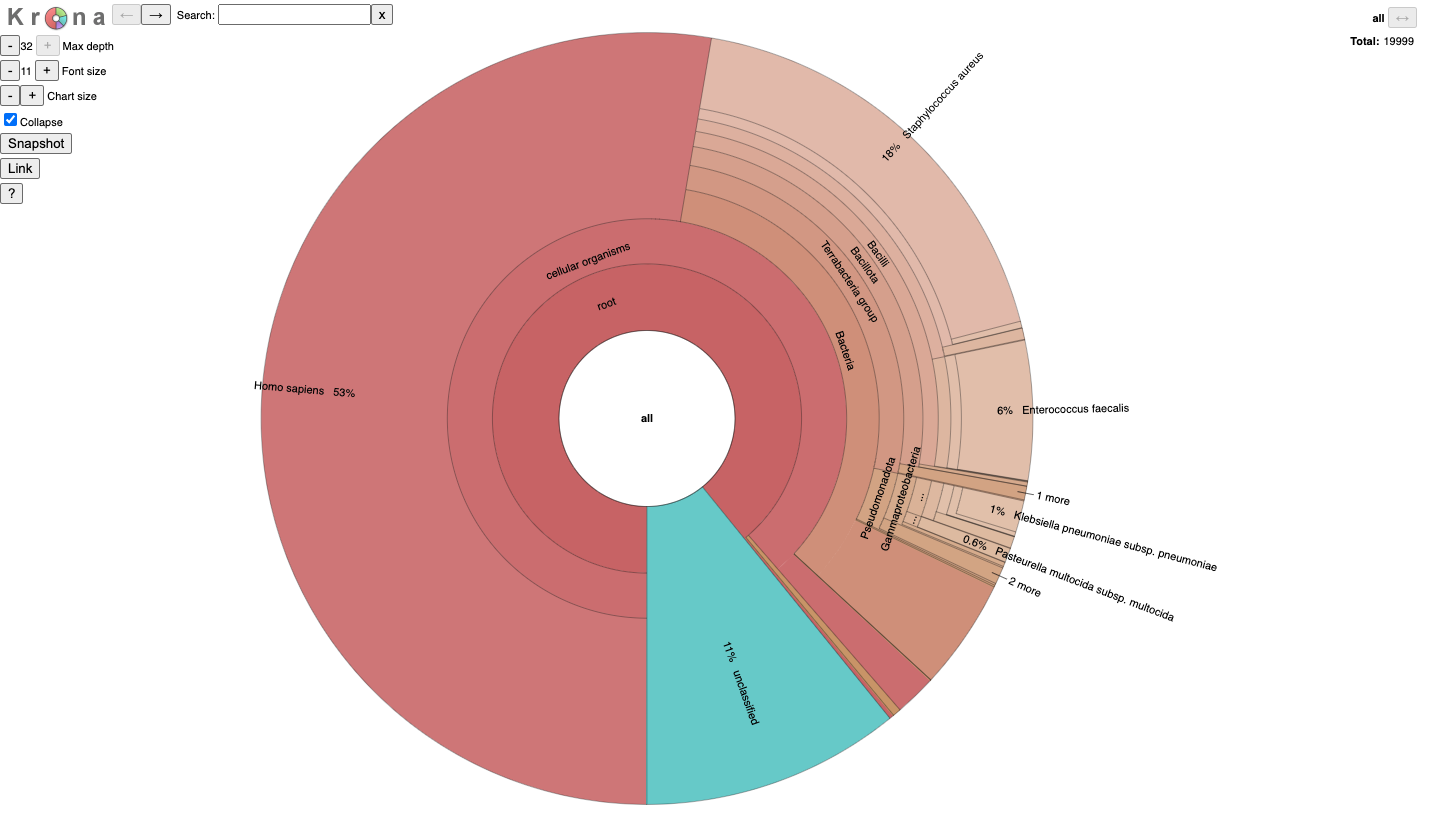
#### JobID_krona.html
It is for an interactive taxonomy report (Krona). You can use any modern web browser to open `JobID_krona.html`.
### Resource requirements
Metabuli can classify reads against a database of any size as long as the database is fits in the hard disk, regardless of the machine's RAM size.
We tested it with a MacBook Air (2020, M1, 8 GiB), where we classified about 15 M paired-end 150 bp reads (~5 GiB in size) against a database built with ~23K prokaryotic genomes (~69 GiB in size).
---
## Extract
After running the `classify` command, you can extract reads that are classified under a specific taxon.
This requires the FASTA/Q files used in the `classify` step and the `JobID_classifications.tsv` file, which is generated as one of the output files.
```
metabuli extract --tax-id TAX_ID
- FASTA/Q : The FASTA/Q file(s) used during the `classify` step.
- read-by-read classification : The JobID_classifications.tsv file generated by the `classify` step.
- DBDIR : The same DBDIR used in the `classify` step.
- TAX_ID : The taxonomy ID of the taxon at any rank (e.g., species, genus) from which you want to extract the reads.
# Paired-end
metabuli extract read_1.fna read_2.fna JobID_classifications.tsv dbdir --tax-id TAX_ID
# Single-end
metabuli extract --seq-mode 1 read.fna JobID_classifications.tsv dbdir --tax-id TAX_ID
# Long-read
metabuli extract --seq-mode 3 read.fna JobID_classifications.tsv dbdir --tax-id TAX_ID
```
#### Output
- For paired-end samples: `read_1_TAX_ID.fna` and `read_2_TAX_ID.fna`
- For single-end or long-read samples: `read_TAX_ID.fna`
---
## GTDB-based custom database
> ***User-provided CDS information (optional):***
Use `--cds-info` to provide absolute paths to CDS files. For included accessions, the provided CDS is used, and Prodigal's gene prediction is skipped. Only GenBank/RefSeq CDS files are supported (e.g., GCA_000839185.1_ViralProj14174_cds_from_genomic.fna.gz).
### ***Creat a new database***
>[!IMPORTANT]
>***Requirements***: Reference FASTA file name (or path) must include the assembly accession (e.g., `GCF_028750015.1`, regex`GC[AF]_[0-9]+\.[0-9]+`). Files from RefSeq or GenBank meet this requirement.
#### 1. Download taxonkit-generated GTDB taxdump files [here](https://github.com/shenwei356/gtdb-taxdump/releases).
#### 2. Build
```
# GTDB_TAXDUMP: Directory with downloaded GTDB taxdump files.
# FASTA_LIST: File of reference genome absolute paths.
# DBDIR: Directory where the database will be generated.
metabuli build --gtdb 1 --taxonomy-path [options]
* Options
--threads : The number of threads to utilize (all by default)
--max-ram : The maximum RAM usage. (128 GiB by default)
--accession-level : Set 1 to creat a DB for accession level classification (0 by default).
--cds-info : List of absolute paths to CDS files.
```
This will generate **diffIdx**, **info**, **split**, and **taxID_list** and some other files. You can delete `*_diffIdx` and `*_info` files.
### ***Add new sequences to an existing database***
> [!NOTE]
> If you want to use new GTDB release, please build a new database from scratch.
You can add new sequences to a GTDB-based database. Expanding the taxonomy for virus or eukaryote is also possible.
#### \
```
# GTDB_TAXDUMP: Directory with downloaded GTDB taxdump files.
# FASTA_LIST: File of absolute paths to new sequences.
# NEW DBDIR: Updated database is generated here.
# OLD DBDIR: Directory of an existing database.
metabuli updateDB --gtdb 1 [options]
* Options
--make-library: When many species are in the same FASTA, enable it for faster execution (0 by default).
--new-taxa: List of new taxa to be added.
--threads: The number of threads to utilize (all by default)
--max-ram: The maximum RAM usage. (128 GiB by default)
--accession-level: Set 1 to add new sequences for accession level classification (0 by default).
--cds-info: List of absolute paths to CDS files.
```
#### \
> [!WARNING]
> Mixing taxonomies within the same domain is not recommended. For example, adding prokaryotes to a GTDB database using NCBI taxonomy will cause issues, but adding eukaryotes or viruses using NCBI taxonomy is fine since GTDB does not cover them.
1\. **Check taxdump** files to see if you need to add new taxa. `taxdump` command retrieves taxdump files of an existing database.
2-1\. **Create a new taxa list**
If you have **accession2taxid** and **taxonomy dump** files of the new sequences, you can use `createnewtaxalist` to create an input for `--new-taxa` option. If not, you have to prepare the input manually (see below).
```
metabuli createnewtaxalist
```
It generates `newtaxa.tsv` for `--new-taxa` option and `newtaxa.accession2taxid`.
##### Example
Suppose you're adding eukaryotes to a GTDB database. As GTDB doesn't include eukaryotes, you may want to use NCBI taxonomy for eukaryotes.
You can download `taxdump` files from [here](https://ftp.ncbi.nlm.nih.gov/pub/taxonomy/new_taxdump/) and `accession2taxid` from [here](https://ftp.ncbi.nlm.nih.gov/pub/taxonomy/accession2taxid/).
```
metabuli createnewtaxalist
metabuli updateDB --new-taxa
```
2-2\. **Manually prepare a new taxa list**
For the `--new-tax` option, provide a four-column TSV file in the following format.
```
taxID parentID rank name
```
The new taxon must be linked to a taxon in the existing database's taxonomy.
##### Example
Suppose you want to add *Saccharomyces cerevisiae* to a GTDB database.
After inspecting taxonomy with `taxdump`, you find that the taxonomy lacks the Fungi kingdom and only includes one eukaryote (*Homo sapiens*). In this scenario, your new taxa list and accession2taxid should be as follows.
```
# New taxa list
## taxid parentTaxID rank name // Don't put this header in your actual file.
10000013 10000012 species Saccharomyces cerevisiae
10000012 10000011 genus Saccharomyces
10000011 10000010 family Saccharomycetaceae
10000010 10000009 order Saccharomycetales
10000009 10000008 class Saccharomycetes
10000008 10000007 phylum Ascomycota
10000007 10000000 kingdom Fungi // 10000000 is Eukaroyte taxID of the pre-built DB.
# accession2taxid
accession accession.version taxid gi
newseq1 newseq1 10000013 0
newseq2 newseq2 10000013 0
```
## NCBI or custom taxonomy based database
### ***Creat a new database***
>[!IMPORTANT]
Three requirements:
> 1. **FASTA files** : Each sequence must have a unique `>accession.version` or `>accesion` header (e.g., `>CP001849.1` or `>CP001849`).
> 2. **NCBI-style accession2taxid** : Sequences with accessions absent here are skipped, and versions are ignored.
> 3. **NCBI-style taxonomy dump** : `names.dmp`, `nodes.dmp`, and `merged.dmp`. Sequences with tax. IDs absent here are skipped.
#### 1. Prepare NCBI-format taxonomy dump files and accession2taxid
* Download `accession2taxid` from [here](https://ftp.ncbi.nlm.nih.gov/pub/taxonomy/accession2taxid/).
* Download `taxdump` files from [here](https://ftp.ncbi.nlm.nih.gov/pub/taxonomy/new_taxdump/).
* For custom sequences, edit `accession2taxid` and `taxdump` files as follows.
* `accession2taxid`
* For a sequence whose header is `>custom`, add `custom[tab]custom[tab]taxid[tab]anynumber`.
* As above, version number is not necessary.
* `taxid` must be included in the `nodes.dmp` and `names.dmp`.
* Put any number for the last column. It is not used in Metabuli.
* `taxdump`
* Edit `nodes.dmp` and `names.dmp` if you introduced a new `taxid` in `accession2taxid`.
#### 2. Build
```
# DBDIR: Directory where the database will be generated.
# FASTA_LIST: A file containing absolute paths to FASTA files.
# accession2taxid : NCBI-style accession2taxid file.
# TAXDUMP: Directory with taxonomy dump files.
metabuli build --taxonomy-path [options]
* Options
--make-library: When many species are in the same FASTA, enable it for faster execution (0 by default).
--threads: The number of threads to use (all by default)
--max-ram: The maximum RAM usage. (128 GiB by default)
--accession-level: Set 1 to creat a DB for accession level classification (0 by default).
--cds-info: List of absolute paths to CDS files.
```
This will generate **diffIdx**, **info**, **split**, and **taxID_list** and some other files. You can delete `*_diffIdx` and `*_info` files and `DATE-TIME` folder (e.g., `2025-1-24-10-32`) if generated.
### ***Add new sequences to an existing database***
You can add new sequences to an existing database, of which taxonomy will be used. You can add new taxa if the previous taxonomy does not include them (see "Add sequences of new taxa" below).
#### \
1\. **Prepare two files**
- **New FASTA file list** : Each sequence must have a unique `>accession.version` or `>accesion` header.
- **NCBI-style accession2taxid** : Sequences with accessions absent here are skipped. Put any number in the GI column. Version number is ignored.
```
accession accession.version taxID gi
SequenceA SequenceA.1 960611 0
SequenceB SequenceB.1 960612 0
NoVersionOkay NoVersionOkay 960613 0
```
2\. **Update database**
```
# NEW DBDIR: Directory where the updated database will be generated.
# FASTA_LIST: A file of paths to new FASTA files.
# accession2taxid : NCBI-style accession2taxid file.
# OLD DBDIR: Directory of an existing database.
metabuli updateDB [options]
- FASTA list: A file of paths to the FASTA file to be added.
- accession2taxid : A path to NCBI-style accession2taxid.
* Options
--threads : The number of threads used (all by default)
--max-ram : The maximum RAM usage. (128 GiB by default)
--accession-level : Set 1 to create a DB for accession level classification (0 by default).
--make-library : Make species library for faster execution (1 by default).
--new-taxa : List of new taxa to be added.
```
#### \ - Please refer [this section](#add-sequences-of-new-taxa).
## Example
> The example here was detecting SARS-CoV-2 variant-specific reads, but has changed since the pre-built DB no longer contains the variant genomes.
Classifying RNA-seq reads from a COVID-19 patient.
The whole process must take less than 10 mins using a personal machine.
#### 1. Download RefSeq Virus DB (1.5 GiB)
```
metabuli databases RefSeq_virus OUTDIR tmp
```
#### 2. Download an RNA-seq result (SRR14484345)
```
fasterq-dump --split-files SRR14484345
```
> Download SRA Toolkit containing `fasterq-dump` [here](https://github.com/ncbi/sra-tools/wiki/02.-Installing-SRA-Toolkit)
#### 3. Classify the reads using metabuli
```
metabuli classify SRR14484345_1.fq SRR14484345_2.fq OUTDIR/refseq_virus RESULT_DIR JOB_ID --max-ram RAM_SIZE
```
#### 4. Check RESULT_DIR/JOB_ID_report.tsv
Find a section like the example below
```
92.2331 510490 442 species 694009 Severe acute respiratory syndrome-related coronavirus
92.1433 509993 509993 no rank 2697049 Severe acute respiratory syndrome coronavirus 2
```
## Reference
Shen, W., Ren, H., TaxonKit: a practical and efficient NCBI Taxonomy toolkit, Journal of Genetics and Genomics, https://doi.org/10.1016/j.jgg.2021.03.006